Open disclosure
When an adverse event (AE) occurs, it is needed to inform the patient (or his/her family) about what occurred, its foreseeable consequences, and the alternatives to correct it. There are ethical and clinical reasons for disclosing AEs to patients. Spite of, patients expect to be informed, the attitude of professionals is of excessive caution due to fear that disclosing such information will result in a lawsuit.
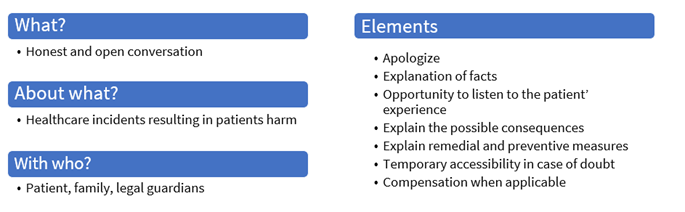
Open disclosure can be defined as open communication with a patient (and/or their support person(s)) regarding a patient safety incident that caused significant harm to the patient while receiving medical care/healthcare. In the case of near misses, it is not recommended to inform the patient. (adapted from Clinical Excellence Commission, 2014)”.1
In practice, only one-quarter of patients receive information after an error that causes them harm. Most healthcare professionals are not properly trained to undertake this communication with a patient.
The way a show of grief should be exhibited for what has happened (an apology) has also been subject to controversy, especially in without apology laws. In recent years, consensus about how to deal with this communication has grown and help tools have been developed.
Disclosing an adverse event to a patient is a complex and distressing situation that requires the following:
- adequate organizational framework
- support from the organization and its management
- knowing what to disclose
- communication skills
- ethical commitment
- a positive attitude that predisposes being open with the patient
Disclosure after an adverse event involves numerous challenges. One of them is how to put disclosure policies and guidelines into real practice; education and training of the healthcare professionals would help. 2 On this subject, the steps to ensure an appropriate disclosure will be detailed in section 08 – How to respond to an AE/incident.
- Clinical Excellence Commission. Open disclosure Handbook. Sidney: Clinical Excellence Commission; 2014.
- Wu AW, McCay L, Levinson W, Iedema R, Wallace G, Boyle DJ, et al. Disclosing adverse events to patients: International norms and trends. J Patient Saf. 2017;13(1):43–9
0 Comments